
H2so4 H2o Reaction Mechanism Dowload Anime Wallpaper HD
Q. In the chemical reaction, K2Cr2O7+xH2SO4+ySO2→K2SO4+Cr2(SO4)3+zH2O the value of x+y+z: Q. In the chemical reaction, K2Cr2O7+XH2SO4+Y SO2→K2SO4+Cr(SO4)3+zH2O, X, Y and Z are respectively, Q. For the oxidation-reduction reaction; K2Cr2O7+XH2SO4+Y SO2→K2SO4+Cr2(SO4)3+ZH2O The values X, Y and Z are: Q.

Potassium dichromate (K2Cr2O7) react with sodium sulfite and sulfuric acid K2Cr2O7+H2SO4
Explanation: The balanced equation is K2Cr2O7 + 3SO2 +H2SO4 → Cr2(SO4)3 +K2SO4 +H2O I think you are referring to the ion-electron method or the half-reaction method. Step 1. Write the skeleton equation The molecular equation is K2Cr2O7 + H2SO4 +SO2 → K2SO4 + Cr2(SO4)3 +H2O
K2Cr2O7 Reaction With H2So4 Carbonyl Compounds chem l help you? K2cr2o7 + h2so4 + c3h7oh
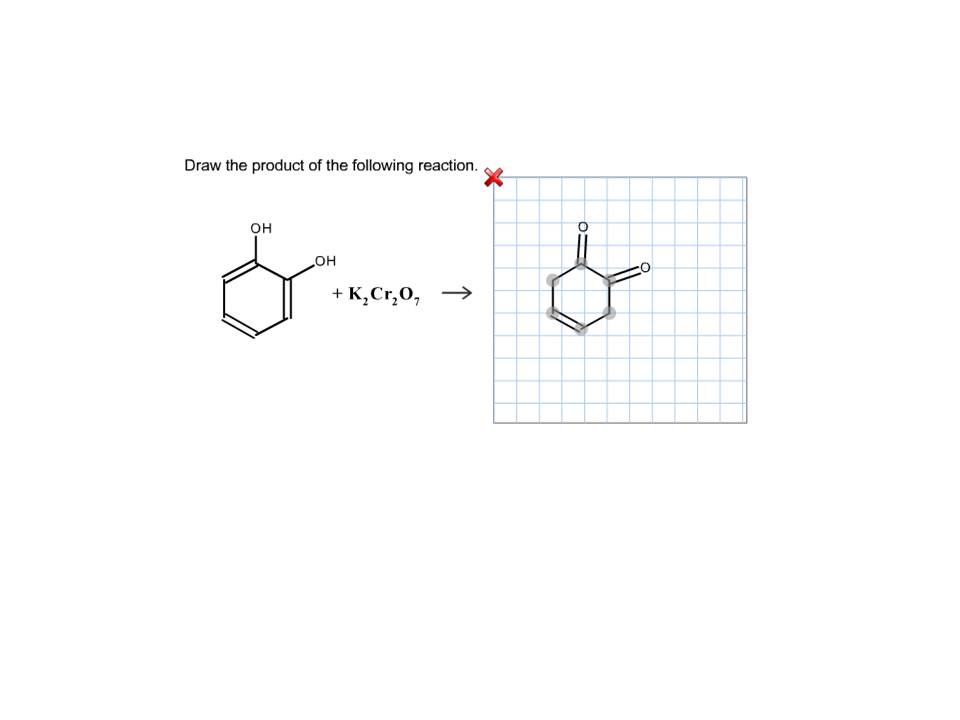
Oxidation by Chromic Acid. One of the reagents that is commonly used for oxidation in organic chemistry is chromic acid. This reagent is straightforward to use once deciphered. However, there are a vast number of different ways that textbooks (and instructors) show it being used in reactions. Chromic acid, H 2 CrO 4, is a strong acid and a.
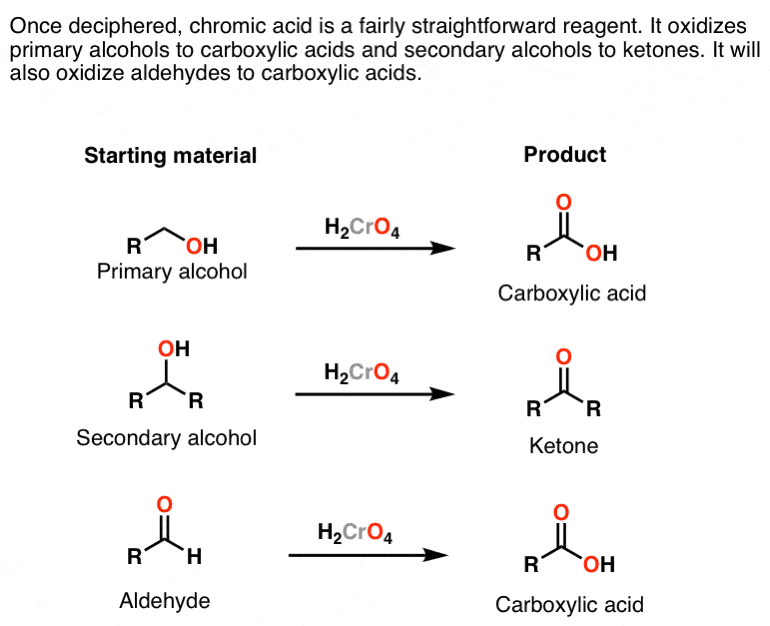
Reagent Friday Chromic Acid, H2CrO4 Master Organic Chemistry
The reaction of it is. K2Cr2O7 + 2KOH → 2K2CrO4 + H2O (here K2Cr2O7 is orange and K2CrO4 is yellow). The reaction is K2Cr2O7 + 4 dil.H2SO4 → K2SO4 + Cr2 (SO4)3 + 4H2O + 3(O) Uses of Potassium Dichromate. Potassium dichromate is used in a large amount in the leather industry. The chrome tanning process involves K2Cr2O7.

Reaction of k2Cr2O7 with H2SO4 Part 61 class 12 unit 8d,f block elements by Vani ma'am YouTube
Balanced Chemical Equation 2 K 2 Cr 2 O 7 + 8 H 2 SO 4 → 2 K 2 SO 4 + 2 Cr 2 (SO 4) 3 + 8 H 2 O + 3 O 2 Warning: One of the compounds in K2Cr2O7 + H2SO4 = K2SO4 + Cr2 (SO4)3 + H2O + O2 is unrecognized. Verify 'Cr2 (SO4)3' is entered correctly. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation.
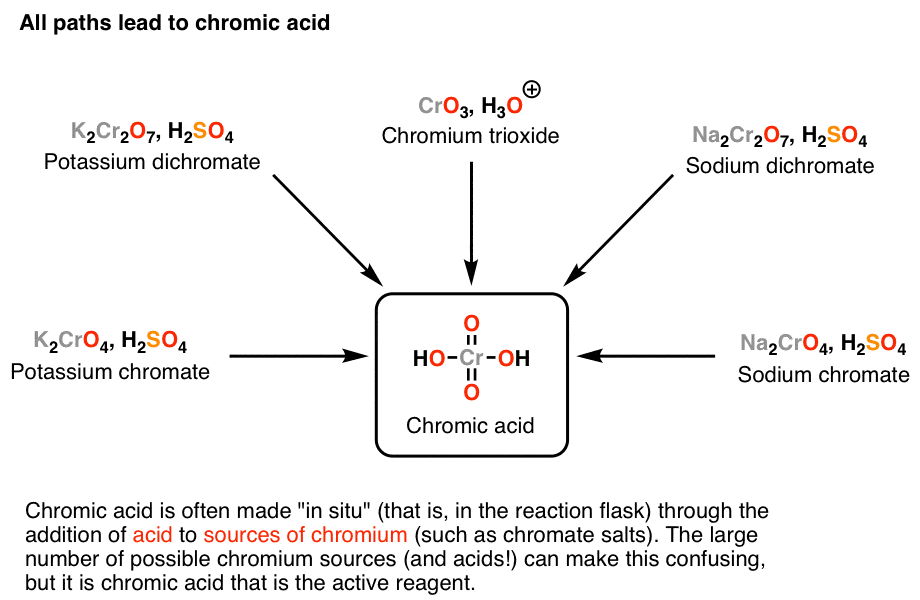
Reagent Friday Chromic Acid, H2CrO4 Master Organic Chemistry
Types of Redox Reactions Question In the given reaction, K2Cr2O7 +XH 2SO4 +Y SO2 → K2SO4 +Cr2(SO4)3 +ZH 2O. Find X, Y and Z. Solution Verified by Toppr SO2 +2H 2O (SO4)2− +4H + +2e−……(1) (Cr2O7)2− +14H + +6e− 2G3+ +7H 2O…(2) Multiplying (1) by 3 and adding (2), 3SO2 +(Cr2O7)2− +2H + 3(SO4)2− +2Cr3+ +H 2O Completing the reaction,

CH3CH2OH+H2SO4+K2Cr2O7=CH3COOH+Cr2(SO4)3+K2SO4+H2O balance the chemical equation by algebraic
4. Balance the number of electrons transferred by multiplying the oxidation half-reaction by 6: 6Fe2+ → 6Fe3+ + 6e-. Step 5/6. 5. Combine the half-reactions and balance the remaining atoms: K2Cr2O7 + 6FeSO4 + 7H2SO4 → 3Cr2 (SO4)3 + 6Fe (SO4)3 + K2SO4 + 7H2O. Answer.
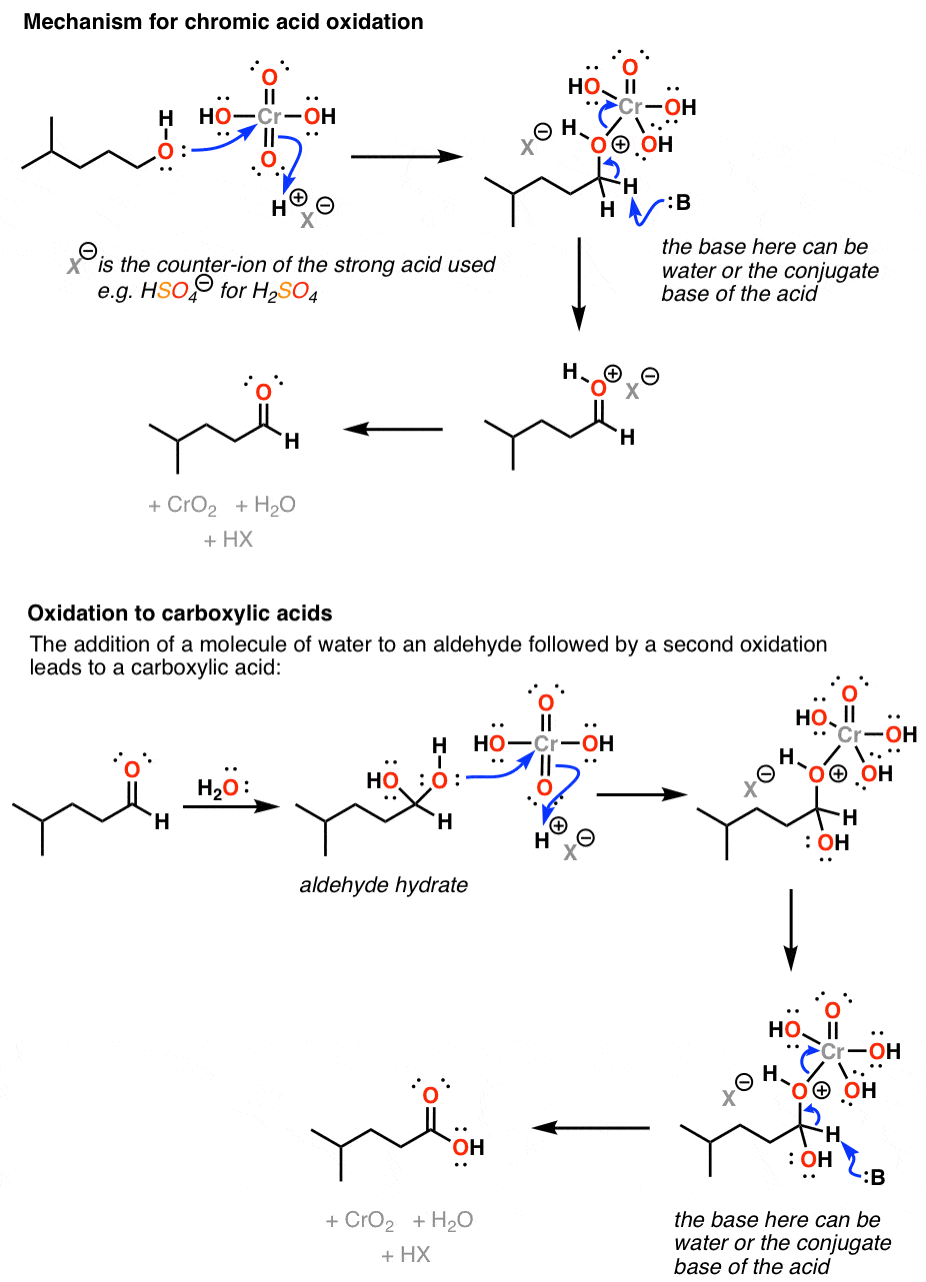
Chromic acid (H2CrO4) as an oxidant in organic chemistry — Master Organic Chemistry
Balanced Chemical Equation K 2 Cr 2 O 7 + -1 H 2 O 2 + 4 H 2 SO 4 → Cr 2 (SO 4) 3 + 3 H 2 O + O 2 + K 2 SO 4 Warning: Negative coefficients mean that you should move the corresponding compounds to the opposite side of the reaction. Warning: One of the compounds in K2Cr2O7 + H2O2 + H2SO4 = Cr2 (SO4)3 + H2O + O2 + K2SO4 is unrecognized.

Chem Expt 3 Reacn. of NaCl & K2Cr2O7 (+ H2SO4) Potassium dichromate, Vapor
10 But I thought why not SOX2 S O X 2 While the reaction is stechiometrically correct, it is hard to perform. In excess of HX2S H X 2 S sulfur will be produced. In excess of oxidant sulfate will be produced. And exact balance is impossible to achieve. That said, SOX2 S O X 2 reacts with HX2S H X 2 S finally producing sulfur.
Solved Draw the major organic product of the reaction shown.
The alcohol is heated under reflux with an excess of the oxidizing agent. When the reaction is complete, the carboxylic acid is distilled off. The full equation for the oxidation of ethanol to ethanoic acid is as follows: 3CH3CH2OH + 2Cr2O2−7 + 16H+ → 3CH3COOH + 4Cr3+ + 11H2O (3) (3) 3 C H 3 C H 2 O H + 2 C r 2 O 7 2 − + 16 H + → 3 C H.

😊 H2o2 k2cr2o7. Redox uncertainty Cr2O7 + H2SO4 + H2O2? chemhelp. 20190226
Reactants: K2Cr2O7 - Potassium dichromate (VI) Other names: Potassium dichromate , Chromium potassium oxide , Dipotassium dichromium heptaoxide. show more Appearance: Red-orange crystalline solid ; Orange-to-red crystals H2O2 - Hydrogen peroxide Other names: Dioxidane , Oxidanyl , Perhydroxic acid. show more

😊 H2o2 k2cr2o7. Redox uncertainty Cr2O7 + H2SO4 + H2O2? chemhelp. 20190226
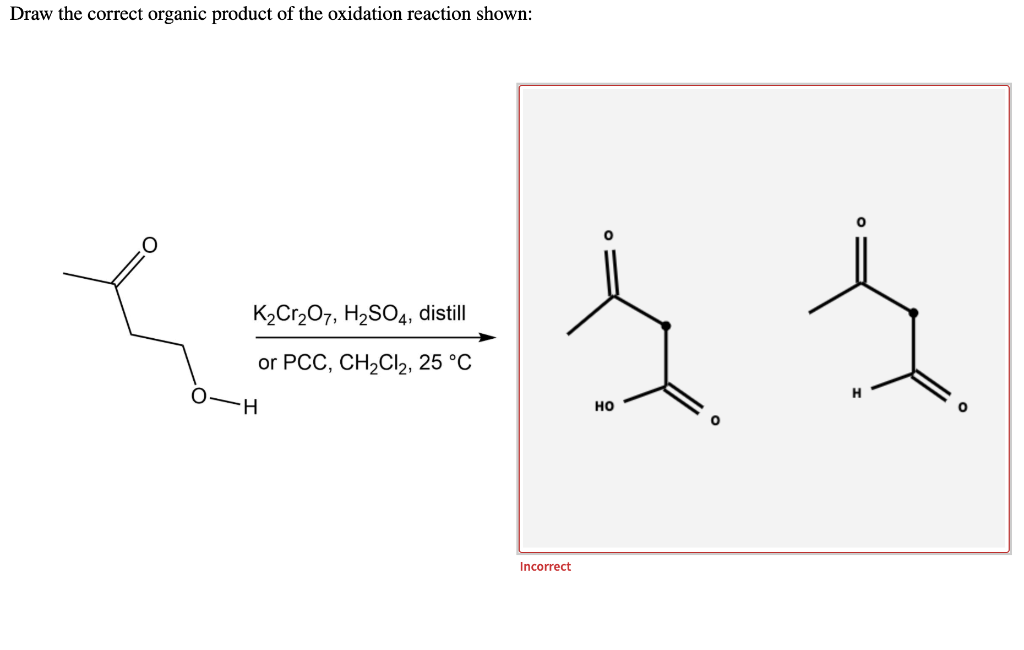
Chemistry Chemistry questions and answers Draw the correct organic product of the oxidation reaction shown: H K2Cr2O7, H2SO4, distill or PCC, CH2Cl2, 25 °C This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
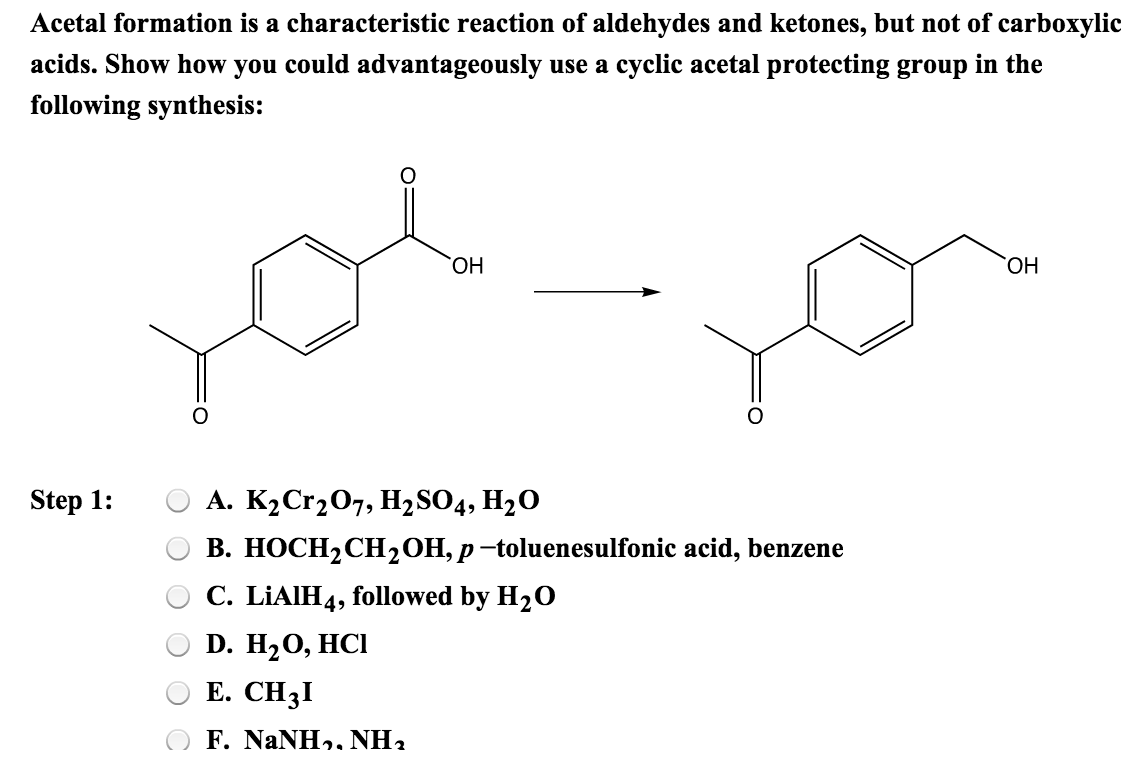
Solved Acetal Formation Is A Characteristic Reaction Of A...
Balanced Chemical Equation 0 K 2 Cr 2 O 7 + 3 H 2 S + H 2 SO 4 → 0 Cr 2 (SO 4) 3 + 0 K 2 SO 4 + 4 S + 4 H 2 O Warning: Some compounds do not play a role in the reaction and have 0 coefficients. Make sure you have entered the equation properly. Warning: One of the compounds in K2Cr2O7 + H2S + H2SO4 = Cr2 (SO4)3 + K2SO4 + S + H2O is unrecognized.
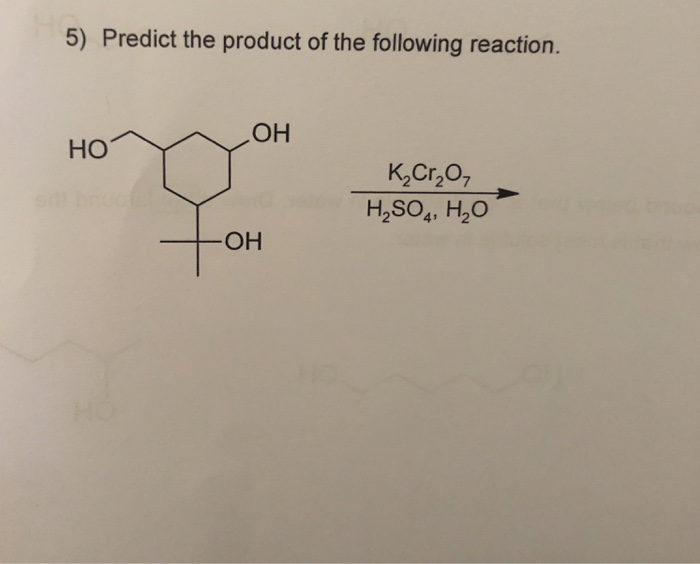
K2cr2o7 H2o Estudiar
H2SO4 | sulfuric acid | solid + K2CrO4 | | solid = H2O | water | solid + K2Cr2O7 | Potassium dichromate; Potassium bichromate; Dichromic acid dipotassium salt | solid
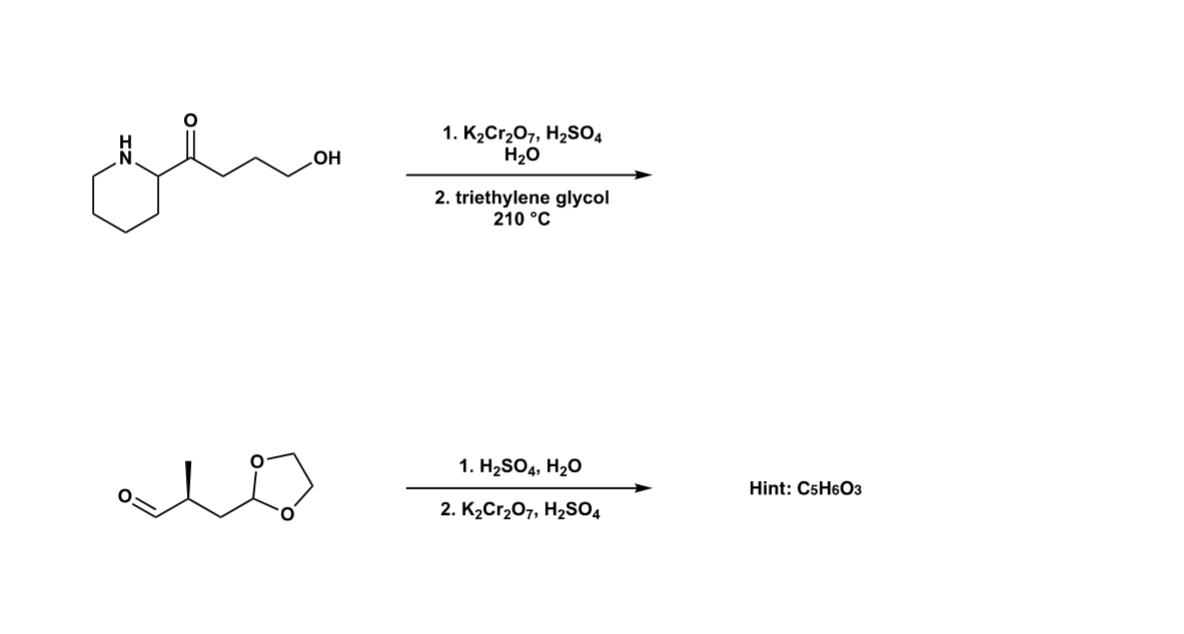
Solved 1. K2Cr2O7, H2SO4 H2O OH 2. triethylene glycol 210 °C
This video is the practical demonstration of the reaction of Acidified Potassium dichromate (k2Cr2O7+H2SO4) with Hydrogen peroxide (H2O2).Precipitation and d.

K2Cr2O7 + H2So4 + Feso4 / Используя метод инноэлектронного баланса,расставьте коэф Don't
Potassium dichromate is usually prepared by the reaction of potassium chloride on sodium dichromate. Alternatively, it can be also obtained from potassium chromate by roasting chromite ore with potassium hydroxide. It is soluble in water and in the dissolution process it ionizes: K 2 Cr 2 O 7 → 2 K + + Cr 2O2− 7 Cr 2O2− 7 + H 2 O ⇌ 2 CrO2−